Join GRAMPUS-CF by following the steps below
1. Download the TrialMotif app.
Set your language/region to English (available regions UK/US/Canada/Australia/Ireland)
2. Open the app and enter your invite code (see ‘step 3’)
3. If the first code you try does not work please try the next one in the list
Invite codes are refreshed and replaced daily.
App invite codes:
GRAMP1-AYPLJQ
GRAMP1-JQ4LRF
GRAMP1-IGJECB
GRAMP1-JLPIHT
GRAMP1-3YZ8UF
4. Consent and register
What does taking part involve?
Once you have read the study information (this link will open a Microsoft Word document) and if you decide to take part, you will be asked to tick the electronic consent on the smartphone app confirming that you understand what is involved.
The app will then ask you to complete a series of questions and questionnaires over 7 days.
We would like you to repeat this process at 6 and 12 months.
Children and young people aged 12-15 years, should only take part with consent from their parent or guardian. The app is not designed for people younger than 12 years. If you are under 12 please do not use the app.
Can I do more to help?
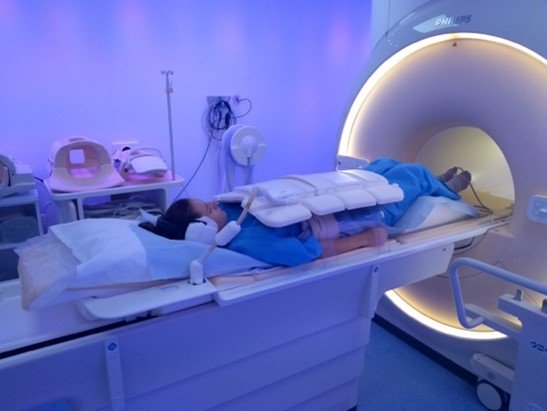
Some UK patients (in Nottingham or Leeds) may want to be part of group B (blood and poo samples) and group C (MRI scan). If you would like to know more about these parts of the study please tick the appropriate box in the consent screen and indicate that you are happy for us to use your email to contact you about this.
Currently only people who attend the Nottingham or Leeds CF centres are able to take part in groups B or C.
Recruitment to groups B and C is limited. Once we reach these numbers will stop recruitment and even if you are willing, we may not be able to take further samples.
What to do next
The first time you use the app, you will also be asked some information about yourself.
Day 1: The app will then ask you to complete a series of questionnaires:
seven GRAMPUS-CF questions (GRAMPUS-7),
a general gastrointestinal symptom measures (GSRS),
a general measure of constipation (PAC-SYM) and
10 questions asking about different tummy symptoms you may have experienced and the impact of these on your daily life over the last 24 hours (CF Tummy Tracker).
Days 2 to 3: We will email you a link to complete a dietary questionnaire (Intake24.org) on the second day. The same link can be used to complete the dietary questionnaire (Intake24.org) again on the following day.
Days 2 to 7: The app will prompt you to complete the 10 CF Tummy Tracker questions
Day 8 : The app will ask you to complete the series of questionnaires from day 1 (GRAMPUS-7, GSRS, PAC-SYM) one last time for this stage.
Follow-up at 6 and 12 months
We would like you to repeat this process at 6 and 12 months. We will send you a reminder to complete the questionnaires via the app at the appropriate times.
The CF Tummy Tracker will also be made available on an optional basis outside of the 6 and 12 months timepoints until the study period is concluded.
What to expect during the study
You are welcome to contact the study team at any point before, during or after the study using the study email address grampuscf@nottingham.ac.uk about any questions you have relating to the study.
The study is otherwise led by the participant which means unless you choose to, you will have minimal direct contact from the study team. You will get prompts and emails from the app and team to help remind you to collect the data.
If you have any technical questions relating to the app itself you can also contact uMotif, who we are working in partnership with at GRAMPUS-CF@umotif.com
What happens after the study
Your participation will end after 12 months. After this time you will still be able to view the answers you recorded on the app, but will not be able to record any more tummy symptoms on it.
We will share our findings on the GRAMPUS-CF website and through social media. We will present our work at CF conferences to share with other researchers and clinical teams and publish our findings in scientific journals