GRAMPUS-CF Online
Please help us to better understand gut symptoms in CF by telling us about about your tummy symptoms every day for 8 days, and what you’ve eaten for 24 hours.
Take part in GRAMPUS-CF Online today from wherever you are in the world.
Exploring gut symptom ‘clusters’ in cystic fibrosis
Many people with CF experience gut symptoms that affect their daily life. Despite the availability of new CF modulator treatments such as Kaftrio/Trikafta, CF gut problems persist in many people.
Not every person with CF experiences the same combination of gut symptoms, so it’s likely there are different mechanisms that cause different combinations. This is what we aim to study.
If we can identify these different ‘clusters’ of symptoms and the mechanisms behind each one, we can develop better, more personalised treatments for people with cystic fibrosis.
GRAMPUS-CF Online is an online study which is part of GRAMPUS-CF.
As part of GRAMPUS-CF participants completed symptom questionnaires, and some also provided stool samples, blood tests and underwent MRI scans. You can find out more about the full study here.
GRAMPUS-CF Online is a related online-only study, where participants complete the same questionnaires used in the main GRAMPUS-CF study, but without the need for in-person procedures.
What does taking part involve?
All you need to do is tell us about about your tummy symptoms every day for 8 days, and what you’ve eaten for 24 hours. This information will help us better understand clusters of tummy symptoms for people with CF. The results will also help in the development of a CF-specific questionnaire to track GI symptoms, CF Tummy Tracker. You can find out more about CF Tummy Tracker here.
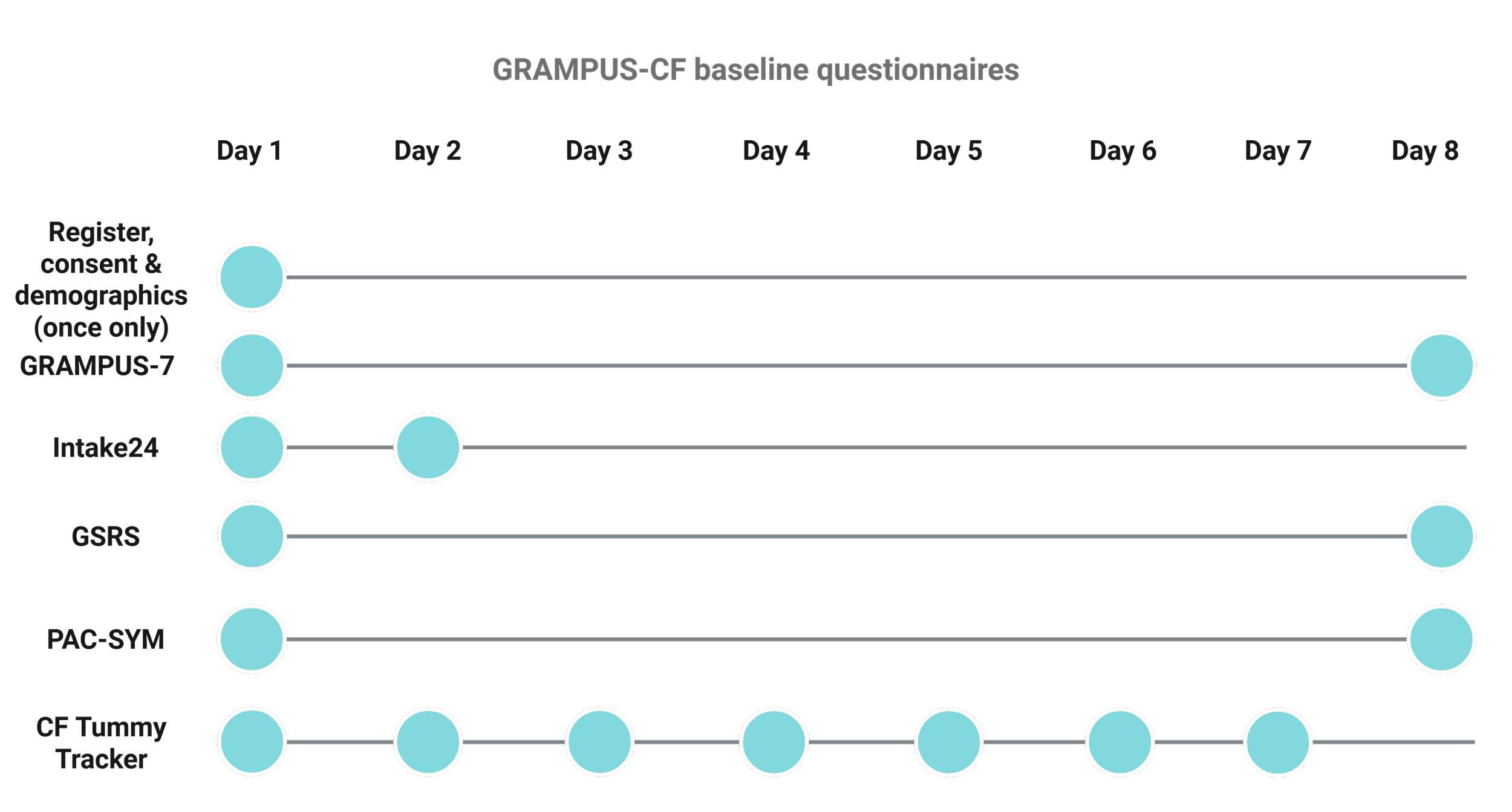
Day 1
First you will be asked some information about yourself and your CF. Then you will need to complete a series of questionnaires:
Seven GRAMPUS-CF questions (GRAMPUS-7),
A general gastrointestinal symptom measures (GSRS),
A general measure of constipation (PAC-SYM) and
Ten questions asking about different tummy symptoms you may have experienced and the impact of these on your daily life over the last 24 hours (CF Tummy Tracker).
Ask you about food and drink consumed in the last 24 hours (Intake 24)
Days 2 to 7: You will receive an email each day to complete the 10 CF Tummy Tracker questions
Day 8: You will receive an email to complete the same series of questionnaires from day 1 (GRAMPUS-7, GSRS, PAC-SYM) again. This will help us to compare your symptoms against what you experienced on day 1.
Follow-up at 6 and 12 months
We understand that circumstances change, but if you are able to, we would like you to repeat this process at 6 and 12 months. We will send you an email to complete the questionnaires at the appropriate times. This is so we can understand if gut symptoms change over time.
Who can get involved?
We want to hear the experiences of as many people as possible with CF.
We are enrolling 150 adults and children (aged 12 years and older) with CF anywhere in the world to take part in GRAMPUS-CF Online.
Children and young people aged 12-15 years, should only take part with consent from their parent or guardian.
Your participation will be helpful whether or not you have trouble with gut symptoms and whether or not you are taking one of the new CF modulator drugs.
Who cannot get involved?
Unfortunately, we cannot enrol anyone with an co-existing bowel condition (not related to your CF) such as crohn’s disease or coeliac disease.
As the online questionnaires are not designed for people younger than 12 years, the study is not suitable for those under the age of 12 years.
What to do next
If you’d like to be involved please follow the steps below to get started
1. Read the participant information sheet
2. Click the button below to go to the GRAMPUS-CF Online study sign up page
GRAMPUS-CF Online recruitment progress
3. Complete screening questions to check you are eligible. Provide your name and a email address
4. Check your email for your link to consent and register. You’re all set!
Study team involvement during the study
You are welcome to contact the study team at any point before, during or after the study using the study email address grampuscf@nottingham.ac.uk about any questions you have relating to the study.
The study is otherwise led by the participant which means unless you choose to, you will have minimal direct contact from the study team. You will get emails from them to help remind you when to provide the data.
What happens after the study
Your participation will end after 12 months.
We will share our findings on the GRAMPUS-CF website and through social media. We will present our work at CF conferences to share with other researchers and clinical teams and publish our findings in scientific journals.
Follow us on Instagram @grampuscf
-
This is an ambitious project and we have brought together a lot of expertise. Here are some of the people who are helping us.
Prof Alan Smyth. Alan is a paediatrician and researcher, based at Queen’s University Belfast. He has spent the last 10 years studying gut symptoms in CF and using MRI scans to do this.
Prof Daniel Peckham. Daniel is a CF chest physician at the University of Leeds. He has previously led a large study of gut symptoms in people with CF and is now bringing his expertise to GRAMPUS-CF.
Prof Luca Marciani and Prof Penny Gowland. Luca and Penny are physicists at the University of Nottingham. They have extensive expertise in performing MRI scans of the gut and interpreting these scans.
Prof Robin Spiller. Robin is a Professor of gastroenterology at the University of Nottingham. Over several decades he has studied gut symptoms and their causes in a wide variety of medical conditions. He is bringing this expertise to CF.
Prof Chris van der Gast. Chris is a microbiologist at Northumbria University. Chris has previously helped us with research which looks at the populations of germs living in the gut of people with CF. Chris is helping us plan and deliver the larger and more ambitious GRAMPUS-CF study.
Dr Tanya Monaghan and Dr Niharika Duggal. Tanya is a gastroenterologist at the University of Nottingham. Tanya studies inflammation in the gut and has a special interest in germs like Clostridium difficile (“C. diff”). Niharika is based at the University of Birmingham and studies inflammation in the lab.
Dr Iain Stewart. Iain works at Imperial College London where he is an expert in statistics. He will help us to crunch the numbers to understand symptom patterns and the underlying causes of these patterns of symptoms.
-
Absolutely. We want to hear from people with a wide range of experiences of gut issues. We will learn a lot from everyone who takes part - whether or not they have troublesome gut symptoms.
-
Taking part in GRAMPUS-CF may not help you directly but we hope that, at the end of the study, we will be able to better understand the reasons behind tummy symptoms in CF. We will then identify suitable treatments to test in clinical trials.
-
At 6 and 12 months you will receive an email asking you to complete the questionnaires again for 8 days. These are the same questionnaires asking questions about gut symptoms, as well as a food diary for 24 hours. This is so we can understand if gut symptoms change over time.
Although it would be helpful to the research to complete these questionnaires at 6 and 12 months, we understand that circumstances might change and you may not be able to. That is ok. Please contribute as much as you are able.
-
During GRAMPUS-CF the questionnaire CF Tummy Tracker will be completed every day for 7 days. It contains 10 questions relating to different aspects of gut symptoms. CF Tummy Tracker was developed by our team as a CF-specific questionnaire to understand tummy symptoms, as most of the questionnaires used in CF are developed for other conditions and so may be less relevant. This study was published earlier this year, and now the results need to be validated as part of GRAMPUS-CF Online. This is to make sure that the questionnaire still works well in a different CF population. You can find out more about this work by visiting www.cftummytracker.org
-
GRAMPUS-CF also involved participants completing the questionnaires used in GRAMPUS-CF Online. In addition, some participants from study sites in the UK (Leeds, Nottingham and Belfast) were also invited to provide blood and stool (poo) samples, and have an MRI scan. This was to provide a complete picture of how people experience gut symptoms, and to try and understand the underlying causes for them. You can read more about GRAMPUS-CF here. Through this work we hope to identify clusters of symptoms which we can then target treatments for, rather than a “one size fits all” approach. GRAMPUS-CF Online forms part of GRAMPUS-CF as we need to understand the patterns of gut symptoms experienced by people with CF and so to do this need to hear the experiences of as many people as possible.
-
Once we understand the clusters of gut symptoms and the underlying mechanisms, our next step will be to test treatments which we will choose based on what we have discovered about the underlying causes. Our ability to do this will depend on funding for a future study.
-
Please email us at grampuscf@nottingham.ac.uk. Your email will be picked up by Darren (Research Dietitian - Nottingham) and Hisham (Medical Research Fellow - Leeds). One of them will get back to you as soon as possible.
GRAMPUS-CF has received generous support from:
The Cystic Fibrosis Trust
The Nottingham NIHR Biomedical Research Centre
The Nottingham Hospitals Charity
Leeds Teaching Hospitals NHS Trust
Motilent
The GRAMPUS-CF Strategic Research Centre is a collaboration between:
The University of Nottingham
Nottingham University Hospitals
University of Leeds
Nottingham Trent University
The University of Birmingham
Imperial College London
Northumbria University Newcastle